Combustion and Flame Class 8 Science Chapter 6 as per NCERT Book used in CBSE and other Schools. The lesson covers the complete explanation of class 8 Chapter 6 Combustion and Flame. Topics covered are combustion, flame and fuels. Difference between types of fuels has been explained. The lesson covers all important questions based on combustion and flame. The lesson also has a look at combustible and non-combustible substances. NCERT book solutions have also been provided for convenience of the students.
| Combustion | Flame | Types of Fuel |
| Types of combustion | Fuel | Global Warming |
| Fire Extinguisher | Acid Rain | NCERT Book Solutions |

The topics we will cover in this chapter are as follows:
- Combustion
- Flame
- Fuels
Introduction
As we know that we need energy to do our routine works. This energy is obtained in the form of food, burning fuels and electricity. This energy is used for cooking, as fuel in vehicles and also in industries. We know that coal and petroleum are non-renewable because they take thousands of years to form. Also, energy is in high demand due to the increase in population and this need is fulfilled by using non renewable resources. Due to this, non renewable resources are in danger as they can get exhausted. So, to overcome this problem, scientists are trying to discover other resources that can be useful in place of non renewable resources.
See Video:
Combustion
Burning of any substance is defined as combustion.
Combustion is defined as a chemical change in which both heat and light are produced at the same time in the presence of oxygen and the composition of substance is also changed.
Example: Burning of coal, paper, candle, etc.
In chemical change, the change that takes place cannot be reversed.
For example: Burning paper converts it into ash and ash cannot be changed back into paper. Physical change is a temporary change and can be reversed and no new substance is formed. For example: If ice melts it can be reconverted into ice without forming any new substance.
Burning of coal also gives energy in the form of light and heat. In this process, the composition of new substance gets changed.
On burning of paper, the composition of ash always gets changed. Oxygen gas is usually used as a supporter of combustion.
Charcoal burns in air to give carbon dioxide and heat.
C + O2 → CO2 + Heat
Methane burns in air and forms carbon dioxide, water and heat.
CH4 + 2O2 → CO2 + 2H2O + Heat
Q. Which out of both, do you think is combustion?
- Burning of coal
- Lighting of bulb
Answer: Burning of coal is said to be a process of combustion. The reason being that in combustion chemical change is necessary, new substance is obtained, it is a permanent change and composition of substance is also changed. So, this occurs only in burning of coal though both produce energy in the form of heat and light.
Conditions required for combustion
The following conditions are necessary for combustion-
- Combustible substance
- Supporter of Combustion that is Oxygen Gas
- Ignition Temperature
Substances can be divided into two parts:
Combustible
Non Combustible
Combustible substances
Substances that easily catch fire are called combustible substances. For example: Kerosene, Petrol etc.
Substances which burn easily are called combustible substances.
Non combustible substances
Substances that do not catch fire easily are called non combustible substances. For example: Glass, Stone etc.
Substances which do not burn in air or oxygen are called non-combustible substances.
Top
Ignition Temperature
It is the lowest temperature at which a substance catches fire. It is different for different substances.
For example: Kerosene oil. If a match stick is burned in a room where kerosene oil is placed, kerosene quickly catches fire. Alternatively, wood does not catch fire on its own at room temperature. But, if wood is heated a little, it catches fire. The only difference between both of them is that Kerosene catches fire quickly, but wood takes time to catch fire.
Q. Out of dry leaves and wet leaves, which catches fire easily?
Answer: Dry leaves catch fire easily because they do not contain water due to which it takes less time to catch fire. Alternatively, in wet leaves due to the presence of water it takes time to catch fire. Water increases the ignition temperature of wet leaves.
Q. Why does petrol catch fire more easily than kerosene?
Answer: Petrol catches fire more easily because its ignition temperature is lower than kerosene. Petrol catches fire with very small amount of heat.
Q. What are inflammable substances? Give example.
Answer: The substances which have very low ignition temperature and can easily catch fire with a flame are called inflammable substances. Combustible substances are also known as inflammable. Examples include petrol, Kerosene, LPG, etc.
Matchsticks
Since ages, matchsticks are in use. Modern matchsticks are made up with mixture of antimony trisulphide and potassium chlorate with some glue and starch applied on the head of the match. The rubbing surface has powdered glass and some red phosphorous. On striking match against rough surface, red phosphorous gets converted into white phosphorous and it reacts with potassium chlorate to ignite antimony trisulphate and so, combustion takes place.
Supporter of combustion
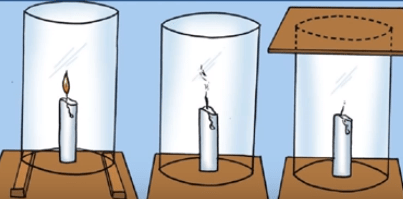
The supporter of combustion is Oxygen gas.
Take a flask and wooden base. Put a candle on the wooden base and cover it with a flask. In this situation, flask is open from which air goes in and out. As we know that air contains oxygen, carbon dioxide, nitrogen, etc. Therefore, it means oxygen gas is inside the flask due to which candle is burning.
In the second step, take another flask and wooden base, but the flask is not open from the top. The top of flask should be covered with cardboard. The air cannot come in from the outside. The air which is inside the flask is used by candle for burning. But if there is no air (oxygen) inside the flask, candle will get extinguished. This is due to absence of oxygen because we cover the flask with cardboard and the oxygen which is present in the flask is already used by the candle.
The chullas used in villages have gaps. These gaps are left between logs of wood for better supply of oxygen. If the oxygen does not enter, proper combustion will not occur. Therefore, we can say that oxygen is a supporter of combustion.
Types of Combustion
There are three types of combustion which are as follows-
- Spontaneous
- Rapid
- Explosive
Spontaneous combustion
It is combustion that occurs at room temperature on its own. It is shown by substances that have an ignition temperature less than room temperature.
For example: Burning of yellow phosphorous. In summers, Yellow phosphorous catches fire itself even at room temperature.
Many disastrous fires in coal mines result due to this kind of combustion. The heat rays coming from the sun or a lightning strike might be responsible for this kind of combustion.
Rapid Combustion
It occurs rapidly, evolving a lot of heat and light in a short period of time. For example: LPG( Liquified Petroleum Gas), Kerosene oil, Coal etc.

Example: Bring a burning matchstick or a gas lighter near a gas stove in the kitchen. Turn on the knob of the gas stove. We find that the gas burns rapidly and produces heat and light.
Coal catches fire rapidly and gives a lot of heat and light. This energy is used for different processes.
Explosive Combustion
It is the combustion that occurs extremely fast with release of heat and light and also loud sound. For example: Crackers.

When a fire cracker is ignited, a sudden reaction takes place with the evolution of heat, light and sound with the large amount of gas.
Q. Forest fires are a result of which type of combustion? Why?
Answer: Forest fires are the result of spontaneous combustion because of the following factors:
- Presence of a combustible substance.
- Presence of right ignition temperature.
- Presence of supporter of combustion in air.
Fire Extinguisher
- Foam type fire extinguisher
- Soda acid type extinguisher
Foam type fire extinguisher
It consists of two cylinders:
Outer cylinder: It is made of metal (contains baking soda).
Inner cylinder: It is made up of glass which contains aluminium sulphate and aluminium sulphate is mixed with saporin.
Both cylinders are bound by common knob. When the knob is pressed, the glass cylinder breaks. Then both chemicals, including baking soda and aluminium sulphates get mixed, react and as a result, produce carbon dioxide that comes out with great pressure, cuts off oxygen supply and fire gets extinguished.
NaHCO3 + Al2(SO4)3 (Aluminium sulphate) → Na2SO4 + Al2(CO3) + CO2 + H2O
Hence, we can say that carbon dioxide is a non supporter of combustion. Saporin which is mixed is useful in formation of Carbon Dioxide and it form Carbon Dioxide in the form of foam.
Soda acid types extinguisher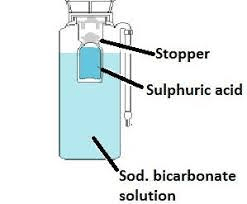
It consists of two cylinders:
Outer Cylinder: It is made up of metal, which contains baking soda.
Inner Cylinder: It is made up of glass which contains sulphuric acid.
Both cylinders are bound by a common knob. When the knob is pressed, the glass cylinder breaks and both chemicals including baking soda and sulphuric acid are mixed, react and as a result, produce carbon dioxide that comes out with great pressure, cuts off oxygen supply and fire gets extinguished. In this process, the carbon dioxide produced is in the form of gas.
Top
Flame
It is defined as the region of burning gases.
When something is burned, a hot luminous gas emerges out of the substance. This gas is called flame. Flames are a result of the substances which vaporize on burning. Example includes kerosene oil, wax, etc. which form flames on burning.
It consists of three zones
- Dark inner zone
- Middle zone
- Outermost zone
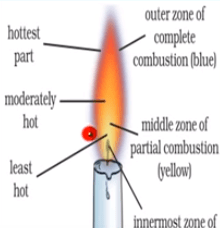
Dark inner zone
- It is black in color.
- It is innermost close to wick.
- It consist of unburnt vapours of wax.
- It is known as no combustion zone.
Therefore, it is least hot.
Middle zone
- It is luminous zone.
- Zone of incomplete combustion. In this zone, partial combustion takes place.
- It is yellow in color.
Therefore, it is somewhat hot.
Outermost zone
- It is the hottest zone.
- It is the result of complete combustion.
- It is the non-luminous zone.
- It is blue in color and faintly visible.
Q. Which part of the flame is used by goldsmiths for melting gold and silver?
Answer: The outermost zone of flame is used by goldsmiths for melting gold and silver. This zone is the hottest part.
Or
Goldsmiths use the outermost zone or non-luminous part of the flame for melting or shaping gold and silver ornaments. They direct the non-luminous part of the flame of candle on the gold with the help of a blow pipe. The temperature of this part of the flame is around 1300°C, it is sufficient to melt gold at specific point and thus helps goldsmiths to give proper shape to the gold ornaments.
Top
Fuel
It is the substance that, on burning, produces a lot of energy.
Or
The substance that undergoes combustion is called fuel. For example: Petrol, Diesel, Coal, wood, charcoal etc.
Uranium is used as fuel in nuclear reactors. It is denoted by “U”. Nuclear energy is very intense energy and obtained from nuclear reactors. Uranium is a radioactive element. Nuclear energy is used for destruction purpose like in formation of bomb. This energy has very harmful effects on health. For example: Hiroshima and Nagasaki incident.
Hydrazine is used as rocket fuel. It is burnt in concentrated nitric acid.
Top
Types of Fuels
Solid fuels: It includes Coke, Coal, Wood etc.

Liquid fuels: It includes Petrol, Diesel, Kerosene.

Gaseous fuels: It includes LPG, CNG etc.

All of these fuels produce energy on burning.
Characteristics of good Fuel
- It should be easy to store and transport.
- It should burn smoothly without emitting harmful gases.
- It should not produce smoke.
- It should be easy to store and transport.
- It should have moderate ignition temperature.
- It should have high calorific value.
Ideal Fuel
The fuel which satisfies all the characteristics of good fuel is termed as an ideal fuel.
Probably, there is as such no ideal fuel present. But liquid and gaseous fuels are better.
Calorific value
It is amount of heat released when 1 gram of fuel is completely burned in sufficient supply of oxygen.
It is measured in kilojoules per gram.
For example: Hydrogen gas.
Q. Which is better fuel out of hydrogen gas, dung cake and LPG ?
Answer: Hydrogen gas is a better fuel as it burns smoothly and produces less smoke. Its calorific value is high and that is 150 kj/gm.
Burning of Fuels
Let us discuss how burning of fuel affects environment.
 Carbon fuels like coal, wood, etc. release a lot of unburnt carbon particles. These particles may enter respiratory tract through air and cause respiratory infection like asthma, bronchitis.
Carbon fuels like coal, wood, etc. release a lot of unburnt carbon particles. These particles may enter respiratory tract through air and cause respiratory infection like asthma, bronchitis.
Incomplete combustion of fuels releases carbon monoxide gas. This gas is very poisonous and harmful and can cause suffocation and even death.
Combustion of most fuels produces carbon dioxide in air. Increased carbon dioxide can cause global warming. More the usage of fuels, more the amount of carbon dioxide present in the air.
For example: In air, there are a lot of gases. Like suppose, there is carbon dioxide in the air. We know that there are three types of rays which comes from sun, that is ultraviolet rays, infrared rays, and visible rays. Ultraviolet rays do not enter into the atmosphere as they get absorbed by the ozone layer. Visible rays are not harmful rays. When infrared rays fall on the earth some of the rays get absorbed and some of them are reflected back and get trapped in the molecules of carbon dioxide. This leads to increase in heat and thereby, rises the temperature of earth.
Top
Global warming
Combustion of most fuels leads to an increase in the amount of carbon dioxide in the atmosphere that has lead to increase in the average temperature on the earth. It leads to ecological imbalance including melting of polar glaciers and causing floods.
Q. What is incomplete combustion?
Answer: Incomplete combustion of a substance takes place in the presence of insufficient amount of air or oxygen. This results in the formation of carbon monoxide, soot, heat & light.
Top
Acid rain
Burning of coal and diesel produces sulphur dioxide gas which can cause suffocation and also it can lead to acid rain.
Or
Due to burning of coal and diesel, Chemicals like sulphur dioxide and nitrogen dioxide are released into the air. The pollutants reacts with the water vapour present in the air and form sulphuric and nitric acid. When it rains, these acids are also present. Such kind of rain is called Acid Rain. It is very harmful for crops, buildings and soil. It also affects aquatic organisms and can cause monument cancer.
Prevention from Acid rain
The use of diesel and petrol as fuels in automobiles is being replaced by CNG (Compressed Natural Gas), because CNG produces harmful products in very small amounts. CNG is a cleaner fuel.
Q. What is the name of substances that can extinguish the paper cup while it catches fire?
Answer: Water, sand, and fire are extinguisher.
Q. Can we boil water in a paper cup while paper catches fire easily?
Answer: Let us take two paper cups. Take water in one cup and keep the other cup empty. Now, heat both the cups. Empty cups start to burn easily while the cup with water does not burn due to an increase in ignition temperature. If we continue heating the water in cup, it starts boiling. This is because heat supplied is transformed to water by conduction. So ignition temperature of water is not reached.
Q. What are the requirements to produce fire?
Answer: Fuel, Air, and Ignition temperature.
Q. What is flame and also write colors of LPG flame and candle flame?
Answer: The burning of vapor forms a flame. The color of LPG is blue and the color of candle is yellow.
Q. Write the list of the conditions under which combustion can take place?
Answer: Combustion is a process of reaction of a substance with oxygen. There are certain conditions required for combustion to take place. They are:
1. Presence of a fuel
2. Air (or oxygen)
3. Ignition temperature (minimum temperature at which a substance catches fire).
Top
NCERT Book Solutions
Multiple choice questions
1. Which substance gives heat and light after combustion
a. Flame
b. Fuel
c. Combustion
d. None of these
2. Like fuel, the sun also provides heat and light. The process taking place in the sun is called
a. Combustion
b. Nuclear process
c. Burning
d. All of these
3. Coal burns with ______
a. Flame
b. Only glow
c. Both flame and glow
d. None of these
4. Burning of charcoal in a closed room will produce
a. Carbon dioxide
b. Nitrogen dioxide
c. Carbon monoxide
d. All of these
5. The substances which have very low ignition temperature will
a. Catch fire easily
b. Will not catch fire
c. Catch fire after some time
d. None of these
6. CNG and LPG are the examples of
a. Solid fuels
b. Liquid fuels
c. Gaseous fuels
d. They are not fuels
7. Ignition temperature is
a. Lowest temperature at catch fire
b. Higher temperature at catch fire
c. Any temperature
d. None of these
8. Combustion is a
a. Chemical process
b. Physical process
c. Both of these processes
d. None of these processes
9. The products of combustion are
a. Carbon dioxide and water
b. Oxygen and water
c. Only carbon dioxide
d. Only oxygen
10. There are following zones of a flame
a. Two
b. Three
c. Four
d. No any zone
ANSWERS
- b
- b
- b
- c
- a
- c
- a
- a
- a
- b
Fill in the blanks:
(a) Burning of wood and coal causes _______ of air.
(b) A liquid fuel used in homes is ___________.
(c) Fuel must be heated to its ___________ before it starts burning.
(d) Fire produced by oil cannot be controlled by ________.
Answer:
(a) Burning of wood and coal causes pollution of air.
(b) A liquid fuel used in homes is liquefied petroleum gas (LPG).
(c) Fuel must be heated to its ignition temperature before it starts burning.
(d) Fire produced by oil cannot be controlled by water.
Q. Explain how the use of CNG in automobiles has reduced pollution in our cities?
Answer: Combustion of fuels like petroleum causes formation of unburnt carbon particles along with carbon monoxide gas. These harmful pollutants enter the air and cause respiratory diseases. Compressed Natural Gas (CNG) produces these harmful products in very less quantity. It is a comparatively cleaner fuel. Therefore, the use of CNG has reduced pollution in our cities.
Q. Compare LPG and wood as fuels?
Answer: Wood has been a traditional fuel for both domestic and industrial use. However, it produces a lot of smoke that can cause respiratory problems. Also, wood is obtained from trees. Thus, using wood as a fuel causes deforestation. Therefore, wood is being replaced by LPG, which is a liquefied form of petroleum gas. It does not give out smoke and other pollutants and is a cleaner fuel. Fuel efficiency of LPG is more than that of wood. The calorific value of LPG is 55000 kJ / kg, while that of wood is between 17000 to 22000 kJ / kg. Hence, LPG is favored over wood.
Give reasons for the following-
- Why is water not used to control fires involving electrical equipment.
- Why is LPG a better domestic fuel than wood?
- Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Answer:
- Water is a good conductor of electricity. The person dousing the fire might get an electric shock if water is used for controlling a fire involving electrical equipments. Also, water can damage electrical equipments.
- LPG is a better domestic fuel as it does not produce smoke and unburnt carbon particles, which cause respiratory problems.
- A piece of paper wrapped around aluminium pipe does not catch fire easily. This is because aluminium, being a metal, is a good conductor of heat. Therefore, heat is transferred from the paper to the metal and the paper does not attain its ignition temperature.
Q. What is the name of the unit in which the calorific value of a fuel is expressed?
Answer: The calorific value of fuel is expressed in kilojoules per kilogram (kJ/kg).
Q. How is CO2 able to control fires? Explain.
Answer: CO2 is a non-combustible gas and extinguishes fire in two ways:
- Since it is heavier than oxygen, it covers the fire like a blanket and cuts off the contact between oxygen and fuel.
- In cylinders, CO2 is kept in liquid form. When carbon dioxide is released, it expands enormously and cools down. This brings down the temperature of the fuel, which helps in controlling the fire.
Q. What is Fuel?
Answer: Any substance which is easily available and burns in air at a moderate rate and produces a large amount of heat energy, without leaving behind any undesirable residue is called fuel. For example: wood, charcoal, petrol, kerosene, etc.
Q. Why sulphur is not used as a fuel even though it can burn in air to produce heat?
Answer: Sulphur is easily available in nature and can burn in air to produce heat. However, it is not a fuel because on burning, it forms a poisonous gas, SO2 , which can cause serious respiratory problems and can even be fatal.
Q. What are the harmful effects of burning fuels?
Answer: The increasing fuel consumption has harmful effects on the environment. The main products formed during the fuel combustion which produce harmful effects are:
- Carbon fuels like wood, coal, petroleum release unburnt carbon particles. These fine particles are dangerous pollutants causing respiratory diseases, such as asthma.
- Incomplete combustion forms carbon monoxide gas. It is a poisonous gas. It is dangerous to burn coal in a closed room. The carbon monoxide gas produced can kill persons sleeping in that room.
- Combustion of most fuels releases carbon dioxide in the environment. Increased percentage of carbon dioxide in the air causes global warming. Global warming is the rise in temperature of the earth. This results in melting of polar glaciers. This leads to rise in sea level and floods in the sea coast.
- Burning of coal and diesel releases sulphur dioxide gas. It is an extremely suffocating and corrosive gas. Sulphur dioxide and nitrogen oxide dissolve in rain water to form acid. Such rain is called acid rain. It is very harmful for crops, buildings and soil.
- Wood is also used as a fuel. Burning of wood gives a lot of smoke which causes air pollution and is also very harmful for humans. It may lead to many respiratory problems. Cutting of trees for obtaining wood Leads to deforestation which is quite harmful to the environment.
- Carbon particles of smoke or ash get suspended in the air. Excessive amount of these in the air causes breathing problems.
Q. Can the process of rusting be called combustion? Discuss.
Answer: Combustion is a chemical process in which a substance reacts with oxygen and gives out energy during the process in the form of either heat or light or both. Rusting of iron is an exothermic process as heat is released during rusting. Hence, it is a kind of slow combustion.
Q. Why is a person caught in fire covered with a blanket?
Answer: If the clothes of a person catch fire, he is immediately wrapped in a thick blanket. The blanket cuts off the supply of air i.e. a supporter of combustion and hence, the fire is put off.
Q: Why does the fire go off when water is poured over burning wood?
Answer: It happens because water absorbs a large amount of heat energy, which results in fall in the temperature of wood below its ignition temperature and the fire goes off.
Note: Fire produced by the burning of oil or petrol cannot be controlled by throwing water on it because water being heavier than oil, settles beneath the oil and oil continues to burn. In the case of fires caused by burning liquid fuels, such as kerosene oil, they can be controlled by throwing sand or soil over it.
Q: Why does a matchstick produce a flame on burning?
Answer: When the matchstick catches fire or is burnt, heat is released due to the burning of chemicals on the match head which partly decomposes the wood to form wood gas. The wood gas then catches fire and produces a flame.
In order to understand the structure of a flame, light a wax candle and watch its flame. Carefully note the different colored zones in the flame. Starting from the base of the flame, a flame has four zones.
(1) Blue zone: It is near the base of the flame. Vaporised wax gets oxidised to form carbon monoxide and carbon monoxide burns completely with a blue flame in this zone.
(2) Dark inner zone: Surrounding the wick is the dark zone. There is no burning in this zone. If we pass a wooden splinter through the dark zone of the flame, it comes out unscratched (unburnt) showing that there is no ‘burning’ in this zone. However, some burnt wax vapors are present in this zone.
(3) Luminous zone: In this region of the flame hydrogen burns with a brilliant yellow luminous flame. Burning hydrogen combines with oxygen to form water vapor. Carbon also burns in this zone giving some luminosity to the flame and producing carbon dioxide. Some unburnt carbon particles are left which give rise to soot.
(4) Outermost non-luminous zone: This zone is poorly visible and is slightly blue. It is the hottest part of the flame where complete oxidation (burning) of the fuel is taking place.
Top