CBSE Class 10 Science Notes of Chapter 5 Periodic Classification of Elements, Video Explanation and Question Answers
Periodic Classification of Elements Class 10 Notes – Periodic Classification of Elements Class 10 Science Chapter 5 as per NCERT Book used in CBSE and other Schools. The lesson covers the complete explanation of Class 10 Science Chapter 5 Periodic Classification of Elements.
Here is a CBSE Class 10 Science Chapter 5 Periodic Classification of Elements Notes and NCERT Solutions to Important Question Answers. Given here is the complete explanation of the chapter, along with examples and all the exercises, Questions and Answers are given at the back of the chapter.
Topics covered are Periodic Classification of Elements Video Explanation, Doberiner’s Traid, Newland’s law of Octaves, Mendeleev’s Classification of Elements, Modern Periodic Table and NCERT Solutions
The lesson covers all important questions and NCERT solutions
Class 10 Science
Chapter 5 – Periodic Classification of Elements
- See Video Explanation of Periodic Classification of Elements
- Dobereiner’s Traid
- Newland’s Law of Octaves
- Mendeleev’s Classification of elements
- Modern Periodic table (prepared by Bohr)
- NCERT Solutions
Introduction to Periodic Classification of Elements
There are many elements known and it is difficult to study their properties separately. So, in order to make their study easier, classification of elements is done.
Classification: is a grouping of similar elements together and separating them from dissimilar ones.
Many attempts have been made by different scientists in order to classify elements. Let us now learn discoveries-
Periodic Classification of Elements Video Explanation
Periodic Classification of Elements Related Links –

According to it, when elements are arranged in order of increasing atomic mass, groups of three elements having similar chemical properties are obtained.
In this, the atomic mass of the middle element is arithmetic mean of atomic masses of the other two elements
Example 1-
| Li | Na | K |
7 23 39 Alkali gp.
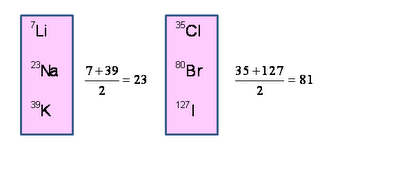
These elements have some chemical properties as follows –
- all are metals
- all react with water to form alkalis
- all have valency-1

Example 2-
| Ca | Sr | Ba |
40 88 137 Alkaline gp.
These elements have some chemical properties as follows –
- all are metals
- oxides of then are alkaline
- all have valency 2.
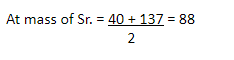
Example 3 –
| Cl | Br | I |
35.5 80 127 halogen gp.
These elements have some chemical properties as follows –
- All are non-metals
- All react with water of form acids
- All have valency 1
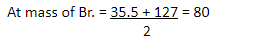
Limitation of Dobereiner’s Triad –
He failed to arrange all known elements in form of triads.

Related – Tips to score 95% in Class 10 Science Paper
Newland’s Law Of Octaves
According to it, when elements are arranged in order of increasing atomic masses, the properties of eighth element are repetition of properties of first element.
He divided the elements into horizontal rows of seven elements such as
| Li | Bi | B | C | N | O | F |
| Na | Mg | Al | Si | P | S | Cl |
| K | Ca |
According to this rule, properties of eighth element i.e. sodium should be similar to those of first element i.e. lithium.
Limitation Of Newland’s Law Of Octaves –
He could classify elements only up to calcium in this way.
Important Videos Links

Mendeleev’s Classification Of Elements
Periodic table is a chart of elements prepared in such a way that elements having similar properties occur in same vertical groups.
According to Mendeleev’s periodic law, Properties of elements are periodic functions of their atomic masses.
| 1 H Hydrogen |
||||||
| 7 Li Lithium |
9 Be Beryllium |
11 B Boron |
12 C Carbon |
14 N Nitrogen |
16 O Oxygen |
19 F Fluorine |
| 23 NA Sodium |
24 Mg Magnesium |
27 Al Aluminum |
28 Si Silicon |
31 P Phosphorus |
32 S Sulphur |
35.5 C1 Chlorine |
| 39 K Potassium |
40 Ca Calcium |
48 Ti Titanium |
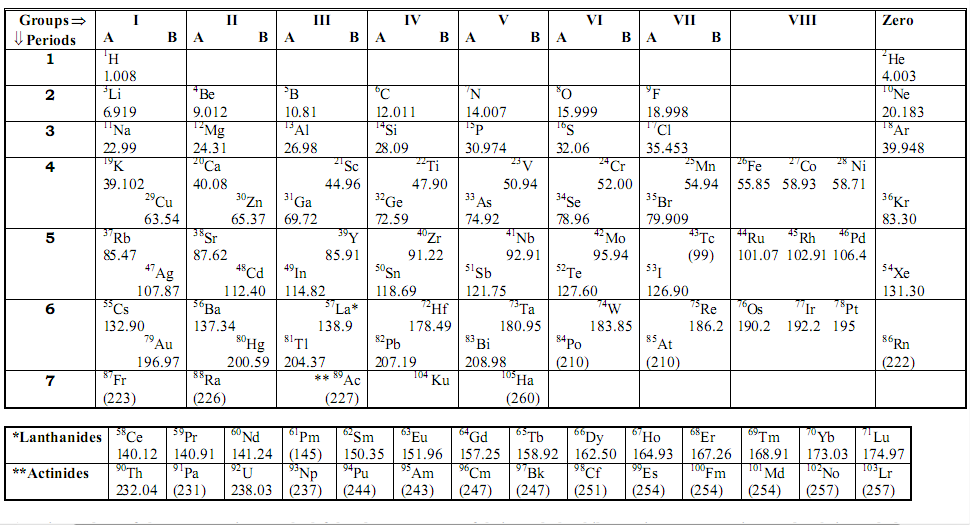
In this, there are seven periods and eight groups. Out of 8 groups, seven are of normal elements and one of transition elements.
He left gaps in his table for unknown elements
He thought that when these elements would be discovered later on, they could be placed in the table without disturbing other elements.
Merits of Mendeleev’s Periodic law are as follows –
1.He grouped the elements on the basis of atomic mass.
2. He left gaps for undiscovered elements like Gallium, Scandium germanium. Also, he left a full group vacant for undiscovered inert gases.
3. He could predict proportions of several elements on basis of their position in periodic table like Ga, Sc, etc.
4.He could predict errors in atomic weights of some elements like gold, platinum, etc.
Anomalies in Mendeleev’s Periodic law are as follows –
1. Position of isotopes could not be explained.
2. Wrong order of atomic masses could not be explained.
For example:- as Arnur atomic mass 40 come first and K with low atomic mass (30) should come later but k should be placed first.
Modern Periodic Table (Prepared By Bohr)

According to Bohr’s Modern Periodic table, properties of elements are periodic functions of their atomic numbers.
Periodic Classification of Elements – So, when elements are arranged according to increasing atomic numbers, there is periodicity in electronic configuration that leads to periodicity in their chemical properties.
It consists of horizontal rows (Periods)
Vertical column (Groups)
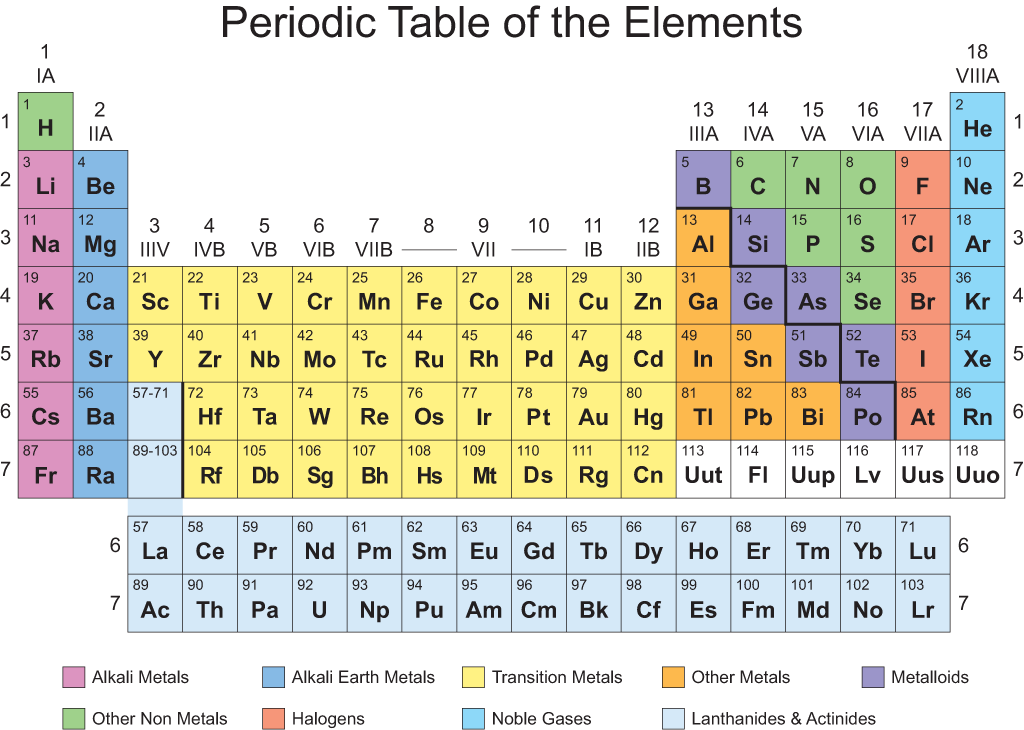
There are 7 period and 12 groups in this long form of periodic table.
Ist period has 2 elements
IInd period has 8 elements
IIIrd period has 8 elements
IVth period has 18 elements
Vth period has 18 elements
VIth period has 32 elements
VIIth period hs rest of elements
Note – The number of valence electrons in atom of elements decides which elements will be first in period and which will be last.
In group:-
1 to 2 gp and 13 to 17 contain normal elements
3 to 12gp – transition elements.
57 to 71 – lanthanides
89 to 103 – Actinides.
Left hand side – metals.
Right hand side – nonmetals.
Note- Hydrogen element has been placed at top of Ist group. Electronic configuration of H is similar to alkali metal as both have 1 valence electron.
V. electron of gp I element — 1
V. electron of gp 2 element — 2
V. electron of gp 13 element — 3
V. electron of gp 14 element — 4
V. electron of gp 15 element — 5
V. electron of gp 16 element –6
V. electron of gp 17 element — 7
V. electron of gp 18 element — 8
Class 10 Science Chapters
Characteristics of periods and groups are as follows –
1. Atomic size is defined as the size of an atom or distance from nucleus to outermost shell of an atom.
Along group – it increases.
For example:- Consider 1st gp
Li – 3 2,1 K,L
Na- 11 2,8,1 K, L, M
K- 19 2, 8, 8, 1 K,L, M, N
As we move down the gp. , every time a new shell is being added due to which size of an atom increases.
Atomic Period decreases.
For example:- consider 3rd period
| Na | Mg | Ar | Si |
| 2, 8, 1 | 2, 8, 2 | 2, 8, 3 | 2, 8, 4 |
We see, every time a new electron is added to the same shell due to which nuclear charge increases and size decreases.
2.Ionization energy – It is the amount of energy required to remove loosely bound electrons from outermost shell of an atom.
Along Group – It decreases.
For example:- consider 1st gp
| Li – 3 | 2, 1 | K, L |
| Na – 11 | 2, 8, 1 | K, L, M |
| K – 19 | 2, 8, 8, 1 | K, L, M, N |
As we move down, every time a new shell is being added due to which nuclear charge decreases and size increases. Less energy is required to remove an electron.
Along Period – it increases.
| Na | Mg | Al | Si |
| 2, 8, 1 | 2, 8, 2 | 2, 8, 3 | 2, 8, 4 |
As we more along period, every time a new electron is added to same shell due to which nuclear charge increases and size decreases. That means nuclear has more attraction for electron and large amount of energy is required.
3. Metallic Character: it is the tendency to lose electrons.
Along Group – it increases
Size increases i.e. valence electrons become more away from nucleus due to which nuclear charge decreases and metallic character increases.
For example –
| Li | Na | K | Rb | Cs | Fr |
| (least metallic) | (most mettalic) |
Along periods – its decreases
From
Na Mg Al Si P S Cl
- Metallic character decreases
- Non-metallic character increases
Size decreases along period due to which Nuclear Charge increases and tendency to gain electrons increases.
4. Nature Of Oxides
Along group – basic oxides increase.
Along Period basic nature of oxides decreases and acidic nature of oxides increases.
5. Chemical Reactivity
Along group – it decreases ( in case of non metals)
It increases (in case of metals)
| F | Decreases | Na | Increases |
| Cl | K | ||
| Br | Rb | ||
| I | Cs | ||
| Fr |
Along Period 🡪 it decreases and then increases
6. Electron Affinity – it is the amount of energy released when electron is added to outermost shell of an atom.

Along Group – It decreases.
For example:- consider 1st gp
| Li – 3 | 2, 1 | K, L |
| Na – 11 | 2, 8, 1 | K, L, M |
| K – 19 | 2, 8, 8, 1 | K, L, M, N |
As we move down, every time a new shell is being added due to which Nuclear charge decreases and size increases. Less energy is released to remove an electron.
Along Period 🡪 it increases.
| Na | Mg | Al | Si |
| 2, 8, 1 | 2, 8, 2 | 2, 8, 3 | 2, 8, 4 |
As we more along period every time a new electron is added to same shell due to which Nuclear charge increases and size decreases. That means nuclear has more attraction for electrons and a large amount of energy is released.
Advantages of Long-form of Periodic table are as follows –
Periodic Classification of Elements of Class 10
- easy to remember and reproduce.
- based on atomic number which is a more fundamental property.
- position of isotopes has been solved.
Periodic Classification of Elements Class 10 Question Answers
Q1. Which of the following statements is not a correct statement about the trends when going from left to right across the periods of periodic table.
(a) The elements become less metallic in nature.
(b) The number of valence electrons increases.
(c) The atoms lose their electrons more easily.
(d) The oxides become more acidic.
A : (c) The atoms lose their electrons more easily.
Q2. Element X forms a chloride with the formula XCl2 , which is a solid with a high melting point. X would most likely be in the same group of the Periodic Table as
(a) Na
(b) Mg
(c) AI
(d) Si
A : Mg
Q3. Which element has
(a) two shells, both of which are completely filled with electrons?
(b) the electronic configuration 2, 8, 2?
(c) a total of three shells, with four electrons in its valence shell?
(d) a total of two shells, with three electrons in its valence shell?
(e) twice as many electrons in its second shell as in its first shell?
A :
(a) neon
(b)Magnesium
(c)silicon
(d)boron
(e)carbon
Q4. (a) What property do all elements in the same column of the Periodic Table as boron have in common?
A : All have 3 as valence electrons
(b) What property do all elements in the same column of the Periodic Table as fluorine have in common?
A : All have 7 as valence electrons
Q5. An atom has electronic configuration 2, 8, 7.
(a) What is the atomic number of this element?
(b) To which of the following elements would it be chemically similar? (Atomic numbers are given in parentheses.) N(7) F(9) P(15) Ar(18)
A : (a) atomic number -17
(b) fluorine (7)
Q6. The position of three elements A, B and C in the Periodic Table are shown below – Group 16 Group 17 – – – A – – B C
(a) State whether A is a metal or non-metal.
(b) State whether C is more reactive or less reactive than A.
(c) Will C be larger or smaller in size than B?
(d) Which type of ion, cation or anion, will be formed by element A?
Ans : (a) A is non metal
(b) more reactive than A
(c) larger than B
(d) it will form anion
Q7. Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
Ans :
| Nitrogen | Phosphorous |
| 2,5 | 2,8,5 |
Out of them nitrogen is more electronegative .
Q8. How does the electronic configuration of an atom relate to its position in the Modern Periodic Table?
A : The valence electrons decide the group of n element and the number of shells decides the period .
Q9. In the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements with atomic numbers 12, 19, 21 and 38. Which of these have physical and chemical properties resembling calcium?
A : The element with atomic number 12 will have physical and chemical properties like Calcium .
Q10. Compare and contrast the arrangement of elements in Mendeléev’s Periodic Table and the Modern Periodic Table.
A :
| Mendeleev periodic table | Modern periodic table |
| It is based on atomic mass | It is based on atomic number |
| He classified only 63 elements | This table has around 114 elements and have more space to occupy by other newly discovered also |
| It has 8 groups and 7 periods | It has 18 groups and 7 period |
| No separate place for lanthanides and actinides | Separately placed lanthanides and actinides |
NCERT Solutions Class 10 Periodic Classification of Elements
Q1. Write electronic configuration of calcium.
A. The electronic configuration of calcium is- 2,8,8,2.
Q2. State modern periodic law.
A. According to the Modern periodic law, the properties of elements are periodic functions of their atomic numbers.
Q3. Name the scientist who performed x-ray studies and confirmed atomic no. as fundamental property.
A. Moseley performed x-ray studies and confirmed atomic no. as fundamental property
Q4. Predict the position of element sodium and fluorine.
A. Sodium has atomic number 11, electronic configuration 2,8,1. So it belongs to 1st group and 3rd period.
Fluorine atomic no. is 9, electronic configuration 2,7 so it belongs to 17th group and 2nd period.
Q5. Out of Cl, S, K, F, Na which belong to same group?
A. The Atomic numbers of the elements are-
| Cl | 17 | 2, 8, 7 |
| S | 16 | 2, 8, 6 |
| K | 19 | 2, 8, 8, 1 |
| F | 9 | 2, 7 |
| Na | 11 | 2, 8, 1 |
So, by looking at their valence electrons we see that Cl and F belong to same group and K and Na belongs to same group and sulphur is the odd one
Q6. Which group is called halogens, chalcogens, rare gases, alkali group, alkaline group?
A. halogens : gp 17
Chalcogens : group 16
Rare gases group : 18
Alakali: group 1
Alkaline: group 2
Q7. Which is most electronegative and electropositive element of periodic table?
A. Fluorine is most electronegative and cesium is most electropositive element of the periodic table.
Q8. Name radioactive elements.
A. Uranium, radium, polonium, thorium are some of the radioactive elements.
Q9. Why inert gases are called inert?
A. They are called inert as they do not react because of their stable electronic configuration
Q10. Name the inert gas which forms certain compounds as an anomalous behaviour.
A. Xenon and krypton form certain compounds like xenon fluoride.
Q11. List few merits of mendeleev’s periodic table.
A. The merits of mendeleev’s periodic table are-
- He grouped the elements on the basis of atomic mass.
- He left gaps for undiscovered elements like Gallium, Scandium germanium. Even he left full group vacant for undiscovered inert gases.
- He could predict proportions of several elements on basis of their position in periodic table like Ga, Sc, etc.
- He could predict errors in atomic weights of some elements like gold, platinum, etc.