Chemical Effects of Electric Currents Class 8 Science Chapter 14 as per NCERT Book used in CBSE and other Schools. The lesson covers the complete explanation of class 8 Chapter 14 Chemical Effects of Electric Currents . Topics covered are Good or Bad Conductors, tester and its Working, LED, complete and incomplete circuit and effects of current in effecting solutions. Also, topics like Electrolyte and Non-electrolytes and electrolysis and applications have been discussed. The lesson covers all important questions and NCERT solutions to book questions have also been provided for convenience of the students.
| classification of substances | incomplete circuit | electroplating |
| good conductors | electrolytes | NCERT book solutions |
| poor conductors | non – electrolytes | |
| Complete circuit | electrolysis |
We all know that our parents always told us not to touch electrical wires, particularly with wet hands. They also told us not to stand under a tree when it is raining and there are thunderstorms. The reason behind this is electric current and its dangerous properties.
Chemical Effects of Electric Current Class 8 Video Explanation
Classification of substances
Current is defined as the flow of electrons in any material.
We come across so many substances but some substances conduct electricity and some do not conduct electricity. The examples of the substances which do not conduct electricity are as follows:
- Rubber
- Glass
- Wood
- Distilled Water

The substances which conduct electricity are computer, refrigerator, oven, television, laptop, music system etc. These are all electric appliances.
We can classify the substances on the basis whether they allow current to pass or not. These are as follows:
- Good Conductors
- Poor Conductors
Good conductors
Good conductors are those conductors that allow current to pass through them. For example: All metals including metal wires, copper wires, aluminium foil, etc.
 Poor conductors
Poor conductors
Poor conductors are those conductors that do not allow current to pass through them. For example: Wood etc.
That is the reason we are not supposed to touch the electric wires while our hands are wet. But we can touch the electric wires with wood as wood is a poor conductor of electricity.
Question: How one can test if the given substance is a good or poor conductor?
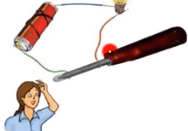 Answer: One can test if a given substance is a good or bad conductor by the use of tester.
Answer: One can test if a given substance is a good or bad conductor by the use of tester.
Tester: It is the instrument that can test whether the given substance is conducting (means it conducts electricity) and non conducting (does not conduct electricity). Then we connect this tester with wires. If bulb glows, it means that it conducts electricity, but if it does not glow, it means that it doesn’t conduct electricity and the given substance is not a conductor.
Question: Let us perform an activity showing how to check that a tester is working or not?
Answer: To perform this activity, we firstly need to join free ends of a tester together. This joining will complete the circuit. If the bulb glows, then the tester is working. If the bulb does not glow, it means that the tester is not working.
So far we have tested the solid substances. Let us check whether liquids and air are good or bad conductors.
Question: Do liquids conduct electricity?
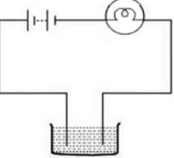 Answer: Let us perform an activity to know whether liquid conduct electricity.
Answer: Let us perform an activity to know whether liquid conduct electricity.
For this, initially we need to take any liquid, say lemon juice in a beaker.
- Then we need to adjust the apparatus by using wires. We need to connect battery through wires to lemon juice container. The bulb is also connected to lemon juice container through wire.
- Then if we see the bulb glows, it means that the given solution conducts electricity.
- Alternatively, if the bulb does not glow, it means that the given solution does not conduct electricity or is non conducting.
- Due to the heating effect of current, the filament of bulb gets heated to a high temperature and it starts glowing. Also, we need to take care that the wires are completing the circuit which means they should not be cut from the middle or any part as this will not allow the bulb to glow.
- But if the current is too weak, the filament does not get sufficient heat and the bulb does not glow. So, there should be appropriate amount of current flowing through the given substance.
Question: How to check the weak current in a circuit?
Answer: There are two types of current which are strong current and weak current. To check strong currents, we can use tester. Strong current leads to glowing of bulb and this also means that current flows through it. There are certain substances which conduct electricity, but the amount of the current present in it is low. To check weak currents, we use LED, that is Light Emitting Diode.
LED has a small bulb. There are two wires called leads attached to a LED. One of its lead is longer and other one is slightly short. Longer lead is always connected to the positive terminal of battery called anode and shorter lead is connected to the negative terminal of battery called cathode. If the bulbs glows, this indicates that current is passing through it. Alternatively, if bulb does not glow, this means that there is no current present in the circuit.
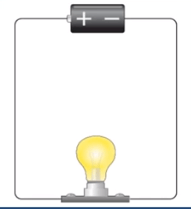 Complete circuit
Complete circuit
Complete circuit means that all connections are proper and there is no break in between them.
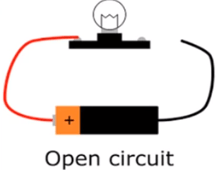 Incomplete circuit
Incomplete circuit
Incomplete circuit indicates the circuit when all the connections are not proper like there may be a break in the wire. This is also called open circuit and current does not flow through it.
Let us perform an activity where we see the major difference between tap water and distilled water.
Tap water: It is good conductor of electricity.
Distilled water: It is poor conductor of electricity.
Let us take two apparatus and in these apparatus we will use batteries and bulbs. These batteries and bulbs are connected to tap water or distilled water through wires. If bulb glows it means that electric current flows through it and we can said that given substance is good conductor. Alternatively, if bulb does not glow that means that there is no electric current which flows through it or we can say that the given substance is bad conductor.
Let us suppose we take A apparatus and put tap water in it. When we set the apparatus, we will see bulb glows. This means that current is flowing through it and the given substance is a good conductor.
In apparatus B, we take distilled water. Then we need to set the apparatus accordingly and we will see that bulb does not glow. This means that there is no current passing through it or the given substance is bad conductor.
Now the question arises in our mind is that we took water in both cases but why only one bulb glows and the another one does not glow. The reason being is that due to presence of impurities, tap water is a good conductor while distilled water is a poor conductor. Also, if we add any acid, iron or any base in water, it makes the water a good conductor.
Question: Let us make a tester using magnetic effect of currents.
Answer: To make a tester using magnetic effect of currents, we need to follow the following instructions-
- Firstly we need an empty tray of a matchbox.
- Then we need to place a magnetic needle in it.
- Wrap an electric wire on it.
- When we join the free ends, we will notice that the needle shows deflection. This deflection is due to magnetic effect.
- Now, connect two free ends of wire with a terminal of battery.
- Now it is ready to test other substances. If all the circuits are complete, it means it will show deflection and current will pass through it. Alternatively, no deflection takes place in a poor conductor as no current flows through it.
Effect of current on conducting solution
- As we come to know that liquids can conduct electricity. Like lemon juice and tap water are good conductors. But there are few solutions which are bad conductors including distilled water.
- The apparatus used to see the effect of current on conducting solution is electrolytic cell. It contains two electrodes, one is anode which is positively charged and other is cathode which is negatively charged. These two electrodes are placed in a solution. When current passes through the solution, the chemical reaction takes place.
- The occurrence of chemical reactions can be felt either by bubbles of gas on electrodes, changes of colour of electrode or deposits of metal on electrodes.
Check your knowledge by answering the following questions-
Fill in the blanks with correct words –
- Electrolysis is used for ______ one metal over another metal.
- A combination of cells is known as ______ .
- In liquid the moving charges are called ______ .
- The driving force that carries charges around a circuit is ______ force.
- Electric current is the flow of negatively charged particles called ______.
- An electric current can bring about a ______ change.
- An ______ when dissolved in water, breaks up into ions.
- ______ are materials that allow electricity to flow through them.
- ______ are also called as insulators.
- A source of electricity is called a ______ .
Answers
-
- Electroplating
- Battery
- Ions
- Electromotive
- Electrons
- Chemical
- Electrolyte
- Conductors
- Non- conductors
- Cell
Classification
The solutions can also be classified on the basis of whether the solution undergoes chemical change or not are written as follows:
- Electrolytes
- Non electrolytes
 Electrolytes
Electrolytes
Electrolytes are those substances which, on passing current dissociate into ions. For example: Solutions of acids bases and salts. Let us take an example of hydrochloric acid.
HCl → H+ + Cl-
 Non-Electrolytes
Non-Electrolytes
Non-electrolytes are the substances which do not dissociate into ions when current is passed through them. For example: Distilled water.
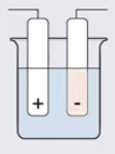 Electrolysis
Electrolysis
Electrolysis is the process in which an electrolyte dissociates into its respective ions when the current is passed through it. Let us take an example:
AB → A+ + B-
This complete electrolysis occurs in a cell called electrolytic cell. This contains a container in which two electrodes are placed. These electrodes are connected to the cell or battery through the wires. One electrode is positively charged and known as anode. The other electrode which is negatively charged is known as cathode. The positive terminal of the battery is connected to the anode (positively charged) and negative terminal of the battery is connected to the cathode (negatively charged electrode).
Electrode: It is a source of current for solution.
 Cathode: It is a negative terminal.
Cathode: It is a negative terminal.
Anode: It is a positive terminal. The electrodes are generally made up of carbon (graphite).
Electrolyte: A substance or solution placed in the container in which dissociation of ions takes place. The chemical reactions take place and as a result various observations can be seen.
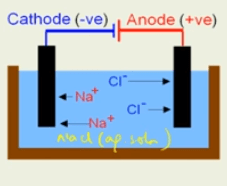
Electrolysis of aqueous solution of sodium chloride
As we know that cathode is a negative terminal and anode is a positive terminal. Here the electrolyte we are going to use is sodium chloride solution. On passing current it dissociate into ions.
NaCl → Na+ + Cl-
Following reaction takes place-
- At Anode
Cl- – e- → Cl
Cl + e- → Cl2 (gas)
- At Cathode
Na+ + e- → Na (atom)
These ions are placed in solution. Then, positively charged Na+ migrate towards cathode and negatively charged Cl- migrate towards anode. Then we see that a chemical reaction takes place.
Question: What is the actual direction of electric current?
Answer: Electrons flow from an electron rich body/object to an electron deficit one. In other words, from a body that is negatively charged to a body that is positively charged. However, scientists from earlier days thought that electric current is the flow of positive charge from a positively charged body to a negatively charged body. Therefore, they took this as the direction of flow of current. Today, we consider the flow of conventional electric current to be from positive to negative electrode.
Question: Why is it dangerous to handle electrical appliances with wet hands or while standing on a wet floor?
Answer: Small amounts of mineral salts are present naturally in water. They are beneficial for human health. However, these salts make water a good conductor. So, if we touch an electrical appliance with wet hands or while standing on a wet floor, current will flow
through our body and lead to electric shock.
Question: Define electrolysis.
Answer: If any substance conducts electricity, when dissolved in water and breaks up into its constituents during the process of dissolving, it is known as an electrolyte. The process of breaking down of an electrolyte on passing electric current through it is called electrolysis.
Question: Write one important application of electrolysis in our daily life?
Answer: Electrolysis has many applications in our daily life but the most common of all the applications is electroplating. In the process of electroplating, a thin layer of a metal like gold, silver, chromium, tin, nickel, etc. is coated over another cheaper metal, either to protect the cheaper metal or to make that metal look attractive.
Electrolysis of aqueous solution of water
As we know that cathode is a negative terminal and anode is a positive terminal. In this case, the acid is added in water and it is used as an electrolyte. Then we need to place two inverted tubes in the container. Electrolyte used in this case is water with little acid. On passing current it dissociates into ions.
H2O → H+ + OH-
Following reaction takes place
We know that now H+ moves towards cathode and OH- will move towards anode.
- At Anode: We will notice oxygen gas bubbles at anode.
- At Cathode: We will notice H2 bubbles at cathode. This indicates that chemical reaction takes place.
H+ + e- → H2 gas
Applications of Electrolysis
- It is used for electroplating.
- It is used for electro refining.
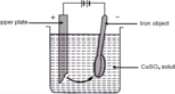 Electroplating
Electroplating
It is the process of depositing metal on desired article. In this process, cathode is the article to be coated, anode is the metal to be deposited, and electrolyte is salt of metal to be coated.
Let us suppose that in the process of electroplating of copper on a metallic spoon, the iron spoon is made the cathode. A thin sheet of pure copper is made the cathode. When electricity is passed through the solution, Cu2+ ion moves towards the cathode and gets reduced to copper metal. This copper metal is electroplated on the object, i.e, metallic spoon.
The sulphate ion moves towards the anode. Here the sulphate ions do not get oxidized. Instead, the copper metal of the anode gets oxidized to Cu2+ ions. These copper ions go into the solution.
Mechanism
On passing current, the electrolytes dissociate into ions. That is
CuSO4 → Cu2+ + SO4 (2-)
These ions migrate to respective poles and chemical reactions occur. As a result, metal gets coated on iron spoon.
 Use of Electroplating
Use of Electroplating
It is used in coating metal with different metals so as to improve its properties.
Example: Chromium plating is generally done on taps etc because it resists corrosion and scratches etc.
Jewellery makers electroplate silver and gold with less expensive metals.
Tin cans used for storing are electroplated with tin on iron.
Zinc is coated on iron is used in bridges, automobiles so as to prevent corrosion.
NCERT book solutions
State whether the following statements are true or false :-
- Natural water that runs down the hills is 100% pure water
- Formation of a new chemical compound by electricity is electrolysis.
- Kerosene is a non electrolyte.
- Lemon juice is an electrolyte.
- All liquids conduct electricity.
- Passing electric currents through a conducting liquid causes chemical changes.
- Electrolysis is an application of electroplating.
- Vinegar is a conductor of electricity.
- A solution that contains oppositely charged ions conducts electricity.
- Glucose solution is an electrolyte and hence conducts electricity.
- Every ion has both positive as well as negative charges.
- Electricity is a form of energy.
- Asbestos is a good conductor of electricity.
- Current flows in a closed circuit as well as an open circuit.
- Different LEDs may give out light of different colours.
Answers
- False
- False
- True
- True
- False
- True
- False
- True
- True
- False
- False
- True
- False
- False
- True
Question: Does pure water conduct electricity? If not, what can we do to make it a conductor?
Answer: No, the pure water does not conduct electricity. Pure water can be made conducting by dissolving salt in it.
Question: Prepare a list of objects around you that are electroplated.
Answer: Pots of metals, bath taps, ornaments, rims of vehicles, handlebar of cycles and motorcycles, kitchen gas burner, bottom of cooking utensils, handles of doors, tin cans are the some objects around us that are electroplated.
Question: Is it safe for the electrician to carry out electrical repairs outdoors during heavy downpour? Explain.
Answer: No, it is not safe for the electrician to carry out electrical repairs outdoors during heavy downpour. It is because while heavy downpour, there is a high risk of electric shock as water is a good conductor of electricity.
Question: What are the two methods of testing an insulator a conductor?
Answer: The following are the two methods that are used to test an insulator or a conductor.
We often use an electric lamp to test conductivity. An electric lamp is attached to the electric current. If the substance allows the current to pass through it, the bulb will start glowing. Otherwise, the bulb will not glow. But this method does not work when current is small. A magnetic tester is used to test conductivity. When current is allowed to pass through it, it creates a magnetic field and compass shows deflection. The deflection indicates current in the circuit.
Question: What do you mean by electroplating? How does it takes place?
Answer: The process of coating a desired metal on other metal surface by using electric current is called electroplating.
A metal piece and the substance to be coated are dipped in current conducting solution with conducting wires. The object which is to be coated is attached to the negative terminal and the metal to be coated is attached to the positive terminal of battery. When current is passed, the compound solution breaks and free metal particles get deposited on object.
Question: Electroplating is based on
- Magnetic effect of current
- Chemical effect of current
- Heating effect of current
- Physical effect of current
Answer: Chemical effect of current
Question: Poor conductors are
- Plastics
- Wood
- Clothes
- All of the above
Answer: All of the above
Question: Flow of electrons is as
- Electroplating
- Electrodes
- Electrolyte
- Electric current
Answer: Electric current
Question: Adding common salt to distilled water makes it
- Good conductor
- Insulator
- None
- Both
Answer: Good conductor
Question: Electroplating prevents
- Current
- Chemical effect
- Rusting
- All
Answer: Rusting
Question: An electric lamp glows due to
- Chemical effect
- Magnetic effect
- Heating effect
- None
Answer: Heating effect
Question: Describe the specific features of an LED?
Answer: An LED or light emitting diode is an electronic device. It lights up (starts emitting light) even when a very weak current flows through it. It contains two leads, one of which is longer than the other. The longer lead is connected to the cell/battery. Hence, when the current flowing through the circuit is too weak, then, bulb may not glow at all. So, for such cases we can utilise LED which is a more sensitive detector of current.
Question: Chrome plating is very popular in the industry. What are its pros and cons?
Answer: Chrome plated objects have a good lustrous shine and appear attractive. They are scratch and corrosion resistant. Therefore, chromium plating is done on large number of objects, like fancy lights, bathroom accessories, kitchen appliances, automobile
parts etc. However, chrome plating is done in a bath (solution) of chromic acid, which is considered carcinogenic and has been known to be poisonous/toxic and cause sores and ulcers etc.
Question: Are conductors and electrolytes same? If not explain differences between them with examples.
Answer: Materials that allow electricity to flow through them are called conductors. Conductors like, silver, gold, copper, mercury, aluminium, iron, graphite etc are elements, and remain unchanged when they conduct electricity. Substances which conduct electricity when dissolved in water or when melted are called electrolytes. In solution or in molten state, electrolyte decomposes into ions that are charged particles and conduct electricity. Example: common salt, vinegar, caustic soda, etc.
Question: Purification of metals is possible through electrolysis. Describe this application.
Answer: Purification of metals can be done by electrorefining, where metals are refined by electrolytic method. For example: copper, silver, nickel, gold, aluminium etc purified through electrorefining.
The purified metal after electrolysis deposits at the negatively charged electrode i.e. the cathode and is collected from time to time.
Question: List the advantages of electroplating.
Answer: The advantages of electroplating are as follows –
- Electroplating protects surfaces by covering them with a thin layer of metal that will be more resistant to corrosion than the material of which they are primarily composed.
- Gold and silver are excellent conductors of electricity, but they are expensive. Through electroplating techniques, very small amounts of these precious, highly conductive metals can be incorporated into electronic components and integrated circuits. Cell phones, computers and other electronic devices use electroplating techniques in their circuits.
- Tin is deposited on iron cans used as food containers to prevent reaction of food with iron.
- Zinc is deposited on the iron to prevent it from rusting. This process of coating of zinc over the iron article is called galvanization.
- Purification of metals: The chemical effect of electric current is used in the purification of impure metals which is extracted from their naturally occurring compounds called ores.
- Production of metals/ Extraction of metals: Extraction of metals by the process by of electrolysis is known as electro-metallurgy. This process is used in case of highly reactive metals such as sodium.
- Production of compounds: The chemical effect of electric current (or electrolysis) is used in the production of various chemical compounds.
- Decomposition of compounds: The chemical effect of electric current/or electrolysis is used to decompose various chemical compounds into their elements.
Multiple choice questions
1. Which of the following is an insulator?
(a) Human body
(b) Graphite
(c) Pure water
(d) Copper
Answer: Pure water
2. In electroplating, the object to be electroplated is made:
(a) anode
(b) cathode
(c) none
(d) both
Answer: anode
3. The extraction of metals from their ores by electrolysis is called:
(a) electroplating
(b) electrometallurgy
(c) electrorefining
(d) none
Answer: none
4. The refining or purification of metals by passing electric current is called:
(a) electroplating
(b) Electrolysis
(c) Electrorefining
(d) None
Answer: electroplating
5. Carbon rods, used as electrodes are made up of:
(a) Graphite
(b) Wood
(b) Iron
(d) diamond
Answer: Graphite
6. Which of these are the applications of electrolysis?
(a) Metal Extraction
(b) Electroplating
(c) Electrorefining
(d) All these
Answer: Electroplating
7. In an electrolytic cell, the electrode which is connected to the positive terminal of the battery is called:
(a) cation
(b) Anion
(c) Cathode
(d) Anode
Answer: Anion
8. Which of these is plated on the iron to prevent it from chemically reacting with food?
(a) Gold
(b) Silver
(c) Chromium
(d) Tin
Answer: Tin
9. Out of the following, which metal is used to electroplate iron to make it shining and rust resistant?
(a) Chromium
(b) Gold
(c) Zinc
(d) Copper
Answer: Chromium
Fill in the blanks-
- Electroplating is based on the _______ effect of electric current.
- Wood is a________ conductor of electricity.
- Pencil lead is a ________.
- The flow of electrons is called _______.
- Tap water is a ______ conductor of electricity.
- Tap water conducts electricity because it has dissolved ______ in it.
- The process used to purify a metal is called ________.
Answers
- chemical
- bad
- good conductor
- current
- good
- minerals
- electroplating
Question: How can distilled water be made a conductor of electricity?
Answer: There are very few ions in water, so, it does not conduct electricity very well. When table salt is dissolved in water, the solution conducts electricity well because the solution contains ions.
Question: Will a bulb glow when a circuit is completed in an insulating solution?
Answer: No, a bulb will not glow when a circuit is completed in an insulating solution.
Question: What is electroplating?
Answer: Electroplating is a process for coating metal objects with the thin layer of a different metal. It is widely used in various industries.
Question: Vinegar is a sour liquid. State whether vinegar will conduct electricity or not?
Answer: Yes, vinegar will conduct electricity.
Question: Which metal is electroplated on iron for making ‘cans’ used for storing food?
Answer: Tin is deposited on iron cans used as food containers to prevent reaction of food with iron.
